

13 experimentally studied proteins
3 sequences in Swiss-Prot
3,153 unique sequences in UniRef100
Bacterial and archaeal l‑asparaginases
High thermal stability and activity
Dimeric asparagianses from Pyrococcus furiosus and Thermococcus kodakarensis
| Fam ? Class - Clan - Family | Alt ? Alternative historical name / classification | AN ? UniProt accession number | Name ? UniProt entry name, only given here for Swiss-Prot entries | EC | Organism | Cell-Loc | AAs | Structure | PDB | Km i for Asn [mM] | Vmax i for Asn [μmol/min/mg] | Kcat i for Asn [s-1] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-4-11 | - | R4L284 ! Uncertain if sequence and kinetics data match. As presented, the protein lacks the critical conserved Motif 1. | 3.5.1.1 | Bacillus licheniformis | - | 272 | - | - | 0.671 ! As presented, the protein lacks the critical conserved Motif 1 | - | 36.8 ! As presented, the protein lacks the critical conserved Motif 1 | ||
| 1-4-11 | - | A0A6G7ABJ6 | 3.5.1.1 | Bacillus sonorensis | - | 329 | - | - | 2.004 | - | - | ||
| 1-4-11 | - | P26900 | ASPG1_BACSU | 3.5.1.1 | Bacillus subtilis | Cytoplasm | 329 | - | - | 1.579 i Sequence may not be exact match | - | - | |
| 1-4-11 | - | A0A291B5A4 | 3.5.1.1 | Bacillus tequilensis | - | 329 | - | - | 0.070 | - | - | ||
| 1-4-11 | - | A5VMR3 | 3.5.1.1 | Lactobacillus reuteri | - | 329 | - | - | 0.3332 i Sequence may not be exact match | - | - | ||
| 1-4-11 | - | A0A0R2I8I9 | 3.5.1.1 | Limosilactobacillus secaliphilus | - | 333 | - | - | 4.78 | - | 887 | ||
| 1-4-11 | - | Q8TZE8 | ASPG_PYRFU | 3.5.1.1 | Pyrococcus furiosus | - | 326 | Homo dimer / tetramer i Temperature dependent | 5CBP PDBs | 12 | - | 870 | |
| 1-4-11 | PhA | O57797 | 3.5.1.1 | Pyrococcus horikoshii | - | 328 | Homo dimer | 1WLS PDBs | - | - | - | ||
| 1-4-11 | - | F8AHM4 | 3.5.1.1 | Pyrococcus yayanosii | - | 328 | - | - | 6.5 | - | - | ||
| 1-4-11 | - | C5A6T2 | 3.5.1.1 | Thermococcus gammatolerans | - | 328 | - | - | 5.3 | - | - | ||
| 1-4-11 | - | Q5JIW4 | ASPG_THEKO | 3.5.1.1 | Thermococcus kodakarensis | - | 328 | Homo dimer | 5OT0 |
2.6 5.5 |
1121 3300 | 694 | |
| 1-4-11 | - | C6A532 | 3.5.1.1 | Thermococcus sibiricus | - | 331 | - | - | 4.7 | 640 | 400 | ||
| 1-4-11 | - | UPI00029ABCE1 | 3.5.1.1 | Thermococcus zilligii | - | 330 | - | - | 6.08 | - | 3267 |
| Fam ? Class - Clan - Family | Alt ? Alternative historical name / classification | AN ? UniProt accession number | Name ? UniProt entry name, only given here for Swiss-Prot entries | EC | Organism | Cell-Loc | AAs | Structure | PDB | Km i for Asn [mM] | Vmax i for Asn [μmol/min/mg] | Kcat i for Asn [s-1] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-4-11 | - | P26900 | ASPG1_BACSU | 3.5.1.1 | Bacillus subtilis | Cytoplasm | 329 | - | - | 1.579 i Sequence may not be exact match | - | - | |
| 1-4-11 | - | Q8TZE8 | ASPG_PYRFU | 3.5.1.1 | Pyrococcus furiosus | - | 326 | Homo dimer / tetramer i Temperature dependent | 5CBP PDBs | 12 | - | 870 | |
| 1-4-11 | - | Q5JIW4 | ASPG_THEKO | 3.5.1.1 | Thermococcus kodakarensis | - | 328 | Homo dimer | 5OT0 |
2.6 5.5 |
1121 3300 | 694 |
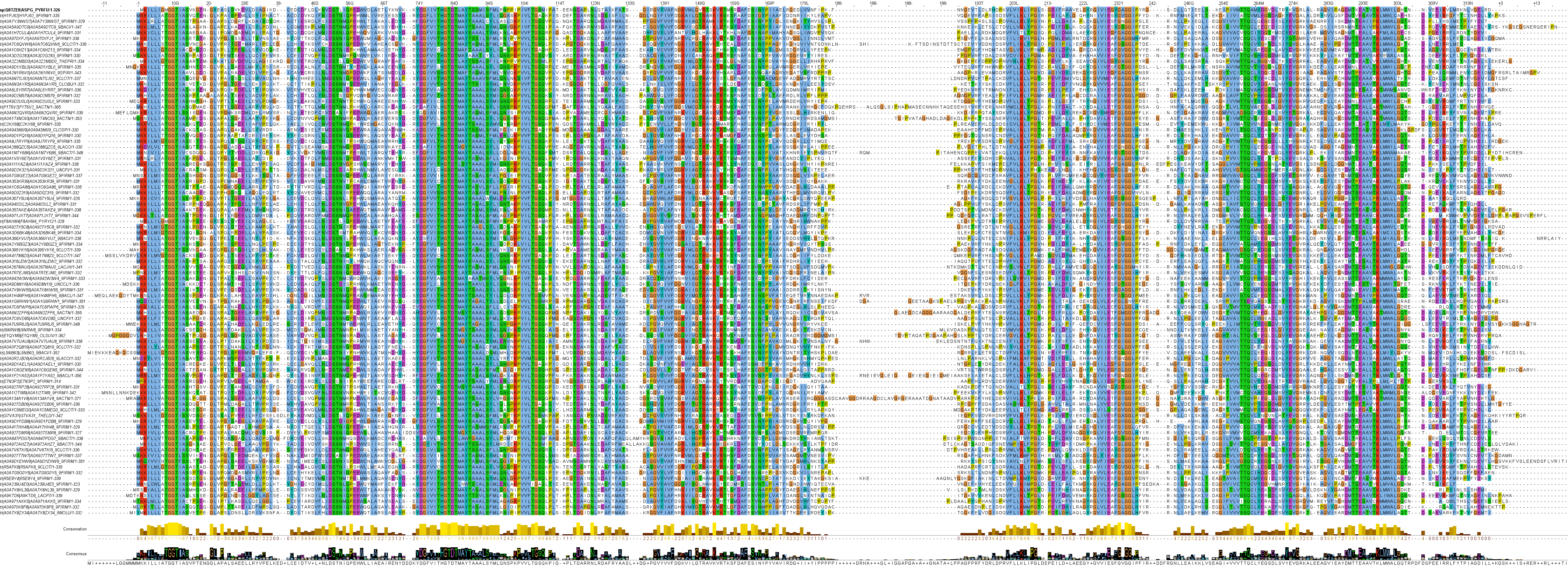
Clan 4 is an interesting clan of bacterial and archaeal l‑asparaginases. Some sequences display characteristics of both "type I" and "type II" l‑asparaginases and some are structurally unique.
Family 11 l‑asparaginases are mostly bacterial, although there are important archaeal representatives present. Three protein structures of l‑asparaginases have been solved in this family, all proteins of archaeal origin: Pyrococcus furiosus PfA (Q8TZE8), Pyrococcus horikoshii PhA (O57797) and the cytoplasmic Thermococcus kodakarensis TkA (Q5JIW4). These l‑asparaginases have a homo dimeric native form and the Km for l‑asparagine tends to be in the millimolar range. These particular l‑asparaginases have likely been chosen for structure analysis due to their archaeal hyperthermophilic origin, but they show high structural similarity (~0.95 TM-score) to numerous bacterial l‑asparaginases in the same family, such as the Bacillus subtilis BsA (P26900).
1Varadi, M et al. AlphaFold Protein Structure Database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Research (2024). Licensed under CC BY 4.0.
2Suzek, B.E. et al. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics (2007). Licensed under CC BY 4.0. Added classification code to sequence headers.
